Research: New Ideas and Instrumentation, Fundamental Plasma Studies, Molecular Analysis and Chemical Sensors, Elemental Mass Spectrometry New Ideas and Instrumentation |
|
Flowing Atmospheric Pressure-Afterglow Array Detector Atomic Mass Spectrometry Design of a Dual Source TOFMS Mass Spectrometer for Speciation Analysis Glow Discharge Atomic Emission Imaging of Gel Electrophoretic Separations Glow Discharges for Liquid Analysis Design and Construction of a Dual Reflectron Time-of-Flight Mass Spectrometer Replacement-Ion Chromatography |
|
|
|
|
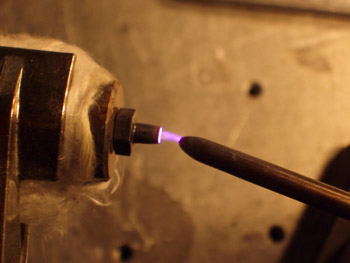
Helium Flowing Atmospheric-Pressure Afterglow (FAPA) Ionization Source |
|
 |
 |
|
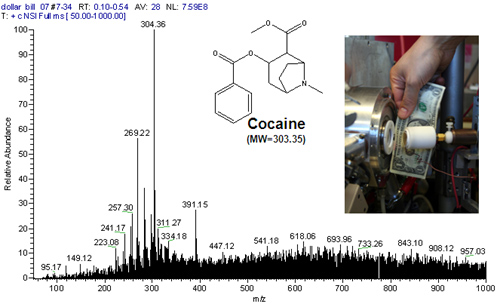
The FAPA is a helium, atmospheric-pressure glow discharge ignited between two electrodes, a pin cathode and a plate or capillary anode, using a direct current power supply. The ions and excited species generated in the discharge are transported by forced convection outside the discharge chamber, where they react with atmospheric constituents to yield reagent ions. When solids or liquids are exposed to the afterglow, analytes are thermally desorbed from the surface and softly ionized with the reagent ions, followed by detection with a mass spectrometer. The resulting mass spectra are very simple, containing either the protonated or radical molecular ion. This approach can be used for the direct analysis of solid, liquid, or gaseous samples with little or no sample pretreatment. Therefore, it has applications in forensic (detection of illicit drugs, explosives, etc.), environmental, and food analysis. Click here for video demonstrating the utility of this source
|
|
|
|
|
|
|
|
|
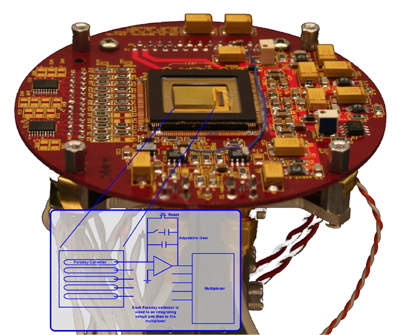
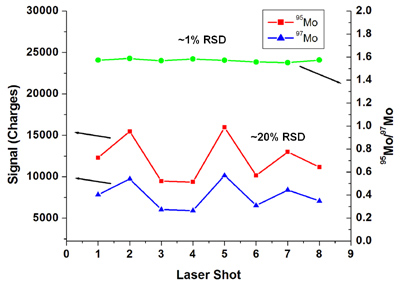
Development of the array detector analytical mass spectrograph (ADAMS) is intended to address significant weaknesses in commercial mass spectrometers. These limitations include duty cycle, transient-signal analysis, and measurement precision. The combination of an array detector and mass spectrograph facilitates simultaneous and continuous detection across the entire active surface of the array. Such detection has been achieved by coupling a Mattauch-Herzog mass spectrograph (MHMS) with a Faraday-strip array detector, the Focal Plane Camera (FPC). The most recent version of the FPC contains 1696 individual ion collectors, each with its own high, variable-gain amplifier. Furthermore, the FPC can be read out at frequencies greater than 2 kHz, which facilitates the detection of rapidly changing signals. Detection of multiple analytes simultaneously, as is accomplished with the MHMS-FPC combination, provides three advantages. First, a much larger portion, theoretically 100%, of the sample is used for the analysis. This high duty cycle provides improved (lower) limits of detection, smaller required sample sizes, and reduced analysis times. Limits of detection are similar to or better than with a commonly used single-channel secondary electron multiplier (SEM). A second advantage is the ability to remove any correlated noise that is imparted on the signal by any of the system components. This is extremely important in isotope-ratio analyses where high-precision measurements are necessary for tracking radioactive waste in the environment, dating geological samples, or monitoring nuclear samples. Finally, simultaneous analyte detection offers more accurate analysis of time-varying signals because each analyte is continuously monitored. This enables more analytes to be accurately monitored within a single chromatographic separation. |
|
|
|
|
|
Design of a Dual-Source TOFMS Mass Spectrometer for Speciation Analysis |
|
 |
 |
|
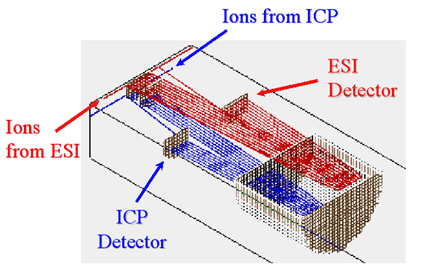
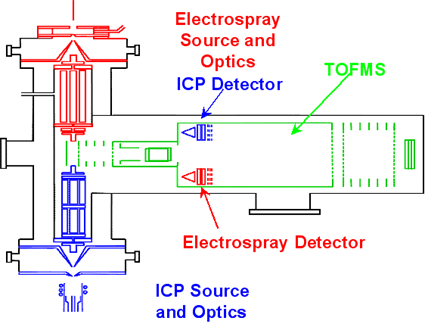
In order to assess the function and toxicity of trace metals in biological and environmental systems, it is necessary to determine not only the trace metals themselves, but also the compound or species within which they exist. Such a process is called speciation analysis, and is commonly done by coupling a separation method, such as chromatography or capillary electrophoresis, with a sensitive elemental detector, such as inductively coupled plasma mass spectrometry (ICP-MS), and separately with a method for structure determination, such as electrospray ionization mass spectrometry (ESI-MS). Element detection and structure determination must currently be performed with separate instruments. The goal of this project is to design and construct an instrument in which both steps occur simultaneously. This "dual source" instrument will combine an ICP source and an ESI source with a single time-of-flight mass spectrometer (TOFMS) in an orthogonal acceleration geometry. The sample will be introduced into both sources at the same time, and a separate detector for each source will provide simultaneous elemental and mass information. |
|
|
Glow Discharge Atomic Emission Imaging of Gel Electrophoretic Separations |
|
 |
 |
|
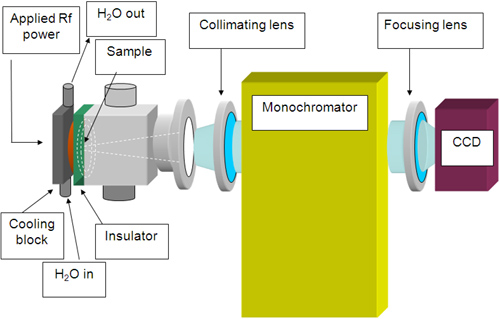
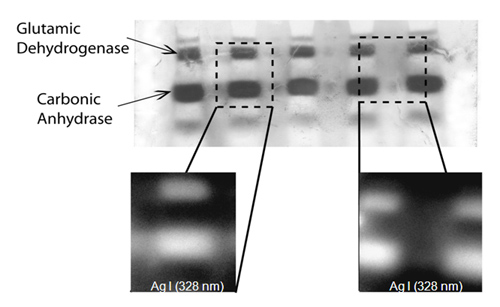
Separations by gel electrophoresis (GE) result in a 2-D spatial map of biopolymers of varying concentration distributed by mass and chemical composition. The analysis of GE separations is often performed by staining or “developing” the gels to reveal the location of each biomolecule. In this project, glow discharge atomic emission spectroscopy (GD-AES) is being explored as a general method to accomplish accurate and highly sensitive protein and biomolecule detection. Previous work in our laboratory has shown that spatial resolution along the surface of a sample can be achieved by using a novel pulsed radiofrequency GD with a monochromatic imaging spectrometer (MIS). When the surface of the sample is imaged at an atomic emission wavelength of interest, monochromatic laterally resolved atomic emission maps are collected, which indicate the identity and quantity of atoms across the sample surface. When the sample is a gel electropherogram, the presence and quantity of proteins or biopolymers can be determined, as can any heteroelements they contain. |
|
|
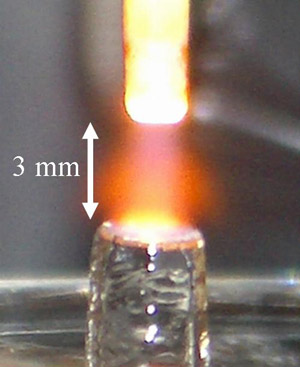
Glow Discharges for Liquid Analysis |
|
 |
 |
|
Traditionally, glow discharges have performed poorly in analyzing liquid samples. We are currently studying two atmospheric-pressure discharges that are able to efficiently atomize and excite the dissolved metals in aqueous solutions. The first, the solution-cathode glow discharge, consists of a glow discharge between a flowing cathode (the sample solution) and a solid anode. Elemental concentrations in the solution are quantified through atomic emission. Because it has a small footprint, uses no compressed gases (i.e. it operates in the open atmosphere), has no narrow apertures to clog, and uses at relatively little power (c. 65 W), it is well-suited for use in field instruments and as a chromatographic detector.
See a video of the ELCAD in use
|
|
|
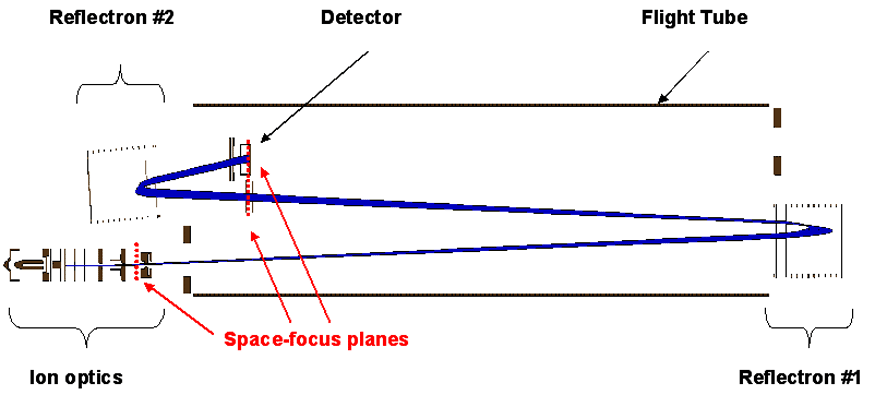
Design and Construction of a Dual Reflectron Time-of-Flight Mass Spectrometer
|
|
 |
|
|
Time-of-flight mass spectrometers typically employ a single reflectron region to compensate for the energy distribution of isomass ions and to improve the overall resolution of the system. When a single reflectron is employed, deflection of the most abundant ions in the ion beam is achieved at the first space focus plane, located immediately after the extraction region. Incorporation of a second reflectron region into the TOFMS design will offer two distinct advantages: an improvement of the resolution of the instrument due to the increase in flight length and more accurate deflection of the unwanted primary species in the ion beam. |
|
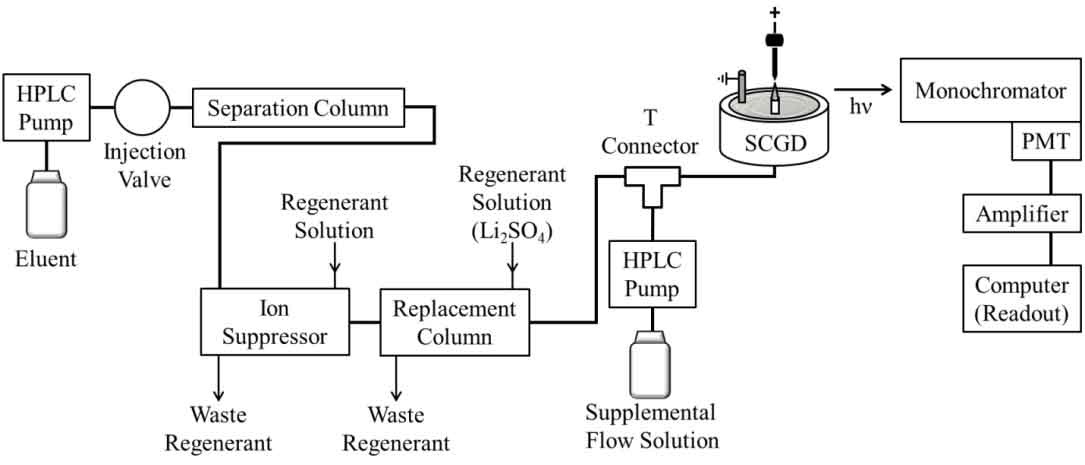
Replacement-Ion Chromatography |
|
Replacement-ion chromatography (RIC) is an alternative scheme for universal ion chromatography (IC) detection in which a third ion-exchange device, referred to as the replacement column, is positioned downstream from the analytical column and ion suppressor of a traditional suppressed-IC system. The replacement column is functionalized with a desired “replacement ion,” so separated analyte ions (or their counter-ions) that enter the replacement column are stoichiometrically exchanged for the replacement ions, which are then monitored downstream with a suitable detector.
|
|